Funding Disclosures
This work was sponsored by Allergan Aesthetics, an AbbVie Company. Writing and editorial assistance was provided to the authors by Regina Kelly, MA, of Peloton Advantage, LLC, an OPEN Health company, and funded by AbbVie. Neither honoraria nor other form of payment was made for authorship.
Author Disclosures
Amy Brideau-Andersen: is an employee of AbbVie, and may hold AbbVie stock
Mitchell F. Brin: is an employee of AbbVie, and may hold AbbVie stock
John Maltman: is an employee of AbbVie, and may hold AbbVie stock
Stephanie Manson Brown: is an employee of AbbVie, and may hold AbbVie stock
Abstract
The potent neuromodulatory effects of the toxin produced by the bacterium Clostridium botulinum were recognized over a century ago when progressive paralytic symptoms of a food-borne illness, botulism, were first documented by a medical officer around the late 18th century. The first therapeutic research use of botulinum toxin, the treatment of strabismus, occurred in 1973. In the 1980s and 1990s, the medical community serendipitously discovered the aesthetic benefits of injecting botulinum toxin into facial muscles, modulating the activity of muscles involved in wrinkle development and smoothing the skin. Today, there are multiple formulations and several known serotypes of botulinum toxin; the 2 commercially available serotypes have distinct medical indications and only type A has cosmetic. All of the botulinum neurotoxins cause temporary muscle paralysis by cleaving proteins involved in the release of neurotransmitters into the neuromuscular junction. This chapter reviews the basic science behind injectable botulinum neuromodulatory agents in facial aesthetics, with an emphasis on pharmacology and mechanism of action.
Introduction
Botulinum neurotoxins (BoNTs) are potent neuromodulators produced by the bacterium Clostridium botulinum1,2 that block vesicular neurotransmitter release and thus inhibit muscle contraction. There are 7 classical, naturally derived BoNT serotypes, identified as A through G.3-5 Serotype A (BoNT/A) has been used in therapeutic applications since the 1970s.6 In the 1980s and 1990s, the medical community serendipitously discovered the aesthetic benefits of injecting botulinum toxin into facial muscles, modulating the activity of muscles involved in wrinkle development and smoothing the skin. Today, the most well-known BoNT/A formulations are those with facial aesthetic indications, which include onabotulinumtoxinA (Botox/Botox Cosmetic/Vistabel/Vistabex), abobotulinumtoxinA (Dysport/Azzalure/Reloxin), incobotulinumtoxinA (Xeomin Cosmetic/Xeomin/Bocouture), and prabotulinumtoxinA (Jeuveau/Nabota/Evosyal/Nuceiva). Here we review the history and basic science behind injectable botulinum neuromodulatory agents in facial aesthetics, with an emphasis on pharmacology and mechanism of action.
Neuromodulator discovery and early aesthetic use
The effects of BoNT on motor and autonomic nerve function were first recognized over a century ago.7,8 Around the late 18th century, a medical officer and poet named Justinus Kerner documented progressive muscle paralysis from a lethal case of botulism, a food-borne illness with symptoms that also included mydriasis, diplopia, and gastrointestinal distress.7,8 An 1895 outbreak of botulism in Belgium resulted in the discovery of Clostridium botulinum by Emile Pierre van Ermengem, a professor of bacteriology.9 Inspired by the discovery of Daniel Drachman in 196510 that chick embryo muscles became paralyzed following BoNT injection, the ophthalmologist Dr. Alan Scott pioneered the use of neurotoxin in 1973 to treat strabismus, which was considered the first therapeutic research use of BoNT/A.11 He named the formulation “Oculinum.”12 Thereafter, in the 1980s and 1990s, small amounts of BoNT/A were used effectively in clinical research to temporarily relax facial and eye muscles to treat hemifacial spasm, strabismus, dystonia, and blepharospasm.7,13 OnabotulinumtoxinA was the first commercially available BoNT/A neuromodulator, having initially received US Food and Drug Administration approval, as Oculinum, to treat strabismus and blepharospasm in 1989.14-16 Oculinum was commercialized by Allergan and the trade name was changed to BOTOX in 1992.17
Patients undergoing treatment for blepharospasm and hemifacial spasm noticed periorbital wrinkles subsiding after injections of onabotulinumtoxinA.18 In 1987, Vancouver-based Drs. Jean and Alastair Carruthers (an ophthalmologist and a dermatologist, respectively) discovered the cosmetic benefits of BoNT/A when their patients who received routine blepharospasm injections reported the smoothing of their upper facial lines.8,18 At the same time, New York-based neurologist Dr. Mitchell Brin and otolaryngologist Dr. Andrew Blitzer discovered similar aesthetic benefits for facial rhytids, especially in the periorbital area, among patients being treated for facial spasms, including hemifacial spasm.19 In 1989, Drs. Clark (plastic surgeon) and Berris (oculoplastic surgeon) reported use of BoNT/A for aesthetic treatment,20 ie, for restoration of facial symmetry in a patient who had a face lift with rhytidectomy complicated by facial nerve damage involving the frontalis.11 Later, in Canada in 2001 and in the United States in 2002, BoNT/A, under the trade name BOTOX Cosmetic,21 became the first neurotoxin approved by regulatory agencies for the treatment of glabellar lines.16
BoNT/A PHARMACOLOGY, MECHANISM OF ACTION, AND NONINTERCHANGEABILITY
Knowledge of the fundamental steps in muscle contraction provides a basis for understanding neuromodulator mechanism of action and pharmacology. Muscles contract in response to signals from the brain passing from peripheral nerve to muscle, triggering an increase in the calcium concentration of the cytoplasm of nerve endings and allowing exocytosis of synaptic vesicles containing the neurotransmitter acetylcholine. Released acetylcholine crosses the nerve junction to activate receptors on muscle fibers, leading to stimulation of muscle contraction.22 A complex of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins enables the process of synaptic vesicle fusion and neurotransmitter release. The neuronal SNARE complex is composed of 3 proteins: synaptosomal-associated protein of 25 kD (SNAP-25), vesicle-associated membrane protein (VAMP, or synaptobrevin), and syntaxin.14,22,23
BoNTs are produced by clostridial bacteria as protein complexes of various sizes, with neurotoxin accessory proteins (NAPs) to protect and stabilize the core 150-kDa neurotoxin.24-28 BoNTs are di-chain molecules, consisting of a heavy (100 kD) chain and a light (50 kD) chain linked by a disulfide bond.5,29 All BoNTs are zinc-dependent metalloproteases1,30; they block the release of acetylcholine at neuromuscular junctions by cleaving SNARE proteins required for vesicle docking and fusion.24 This in turn prevents signal transduction across synaptic junctions and causes chemical denervation, resulting in temporary relaxation of the muscle.20-22 The 7 previously mentioned BoNT serotypes differ on the molecular level and are distinguished by their SNARE substrate specificity, SNARE protein cleavage position, and duration of neuromodulatory effect. BoNT/A, which cleaves the SNAP-25 protein of the SNARE complex, has the longest-lasting neuromodulatory effect and is the most commonly used commercially available subtype.4,5,22 The only other currently commercially available serotype is BoNT/B,22 which cleaves a different protein component of the SNARE complex (VAMP/synaptobrevin) and is approved in the US for the treatment of cervical dystonia and chronic sialorrhea.5,31
Key steps in the neuromodulating effects of BoNT/A on target peripheral muscles include receptor-mediated cell binding and internalization, translocation of the protease domain (light chain) into the cytosol, and proteolytic cleavage of SNAP-25 (Figure 1).30,32,33 Briefly, following injection, BoNT/A is distributed to the extracellular space where the neuromodulator comes into contact with specific receptors along nerve terminals. The heavy chain of BoNT/A binds to these receptors and is internalized via endocytosis. Once in the endosome, the light chain of BoNT/A translocates across the membrane and is released into the cytosol where it cleaves SNAP-25, thereby preventing vesicle fusion with the nerve terminal membrane and, in cholinergic neurons, blocks release of acetylcholine.33,34
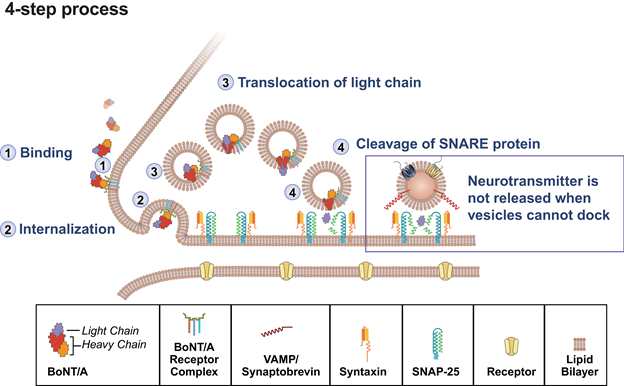
BoNT/A, botulinum toxin A; SNAP-25, synaptosomal-associated protein, 25 kD; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor
Adapted from Burstein R, et al. Cephalalgia. 2014;34(11):853-869.35
Because BoNT products are manufactured, formulated, and tested using techniques that differ across manufacturers,25,36 their preclinical characteristics, clinical properties, and safety profiles may differ, and dosing units of the products are not interchangeable, as stated in product labeling.21,25,37 Understanding the concept of noninterchangeability of BoNT products is important to help ensure adherence to manufacturer labeling and guidance and to inform clinicians on the safe and effective use of these products.
A fundamental reason for the noninterchangeability of BoNT/A products is their biological nature: as bacterially produced proteins, they are much larger with more complex molecular structure than chemically synthesized drugs, and even small modifications to the manufacturing process can alter the protein’s structure and biological activity. In addition to differences among BoNT/A products in several steps of the manufacturing process, each manufacturer employs its own proprietary assay for testing potency units using a product-specific reference standard, meaning that units of biological activity are specific to each product.21,25,38 Several examples of unit noninterchangeability have been confirmed in studies directly comparing the activity of BoNT/A products.37,39-41
Another key difference between products is the presence or absence of NAPs. In nature, bacteria produce BoNTs as protein complexes of various sizes (from 300 to 900 kD for type A) depending on the strain, with NAPs to stabilize and protect the 150-kD neurotoxin from degradation.24-28; Depending on differing purification processes, BoNT/A products either retain one or more of the NAPs in complexes as produced by the bacteria (onabotulinumtoxinA, abobotulinumtoxinA, prabotulinumtoxinA) or only the 150-kD neurotoxin itself (incobotulinumtoxinA).5,16,25,36,42
The translation of these manufacturing and testing differences between BoNT/A products into in vivo differences was suggested by preclinical dose-response studies of muscle weakening efficacy. In these studies, comparison of 3 BoNT/A products reported significant differences in unit potency under the experimental conditions employed.25,40
Overview Of Facial Aesthetic Approved And Potential Indications For BoNT/A
BoNT/A has clinical utility for a variety of neuronal disorders; for example, onabotulinumtoxinA is approved for the treatment of facial wrinkles (glabellar, lateral canthal, and forehead lines) and other medical uses.3 Table 1 provides an overview of BoNT/A neuromodulators presently available and licensed commercially for facial aesthetic indications in the United States and Europe.22 Also shown are any approved indications related to overactive muscle disorders and other medical conditions, where applicable.
Injections of appropriate doses of approved BoNT/A products into forehead, glabellar, and periorbital areas are intended to temporarily improve the appearance of dynamic rhytids in the forehead, glabellar, and crow’s feet regions; additionally, BoNT/A products are sometimes used “off-label” to lift and reshape the eyebrows.20,43-47 Other reported BoNT/A uses include smoothing out grooves and lines in the midface and cheeks.20,48-50 BoNT/A has also been advocated as an effective nonsurgical option for intramuscular injections to treat masseter muscle hypertrophy51 and prominent platysma bands.52
Attributes associated with optimal skin quality have been noted with BoNT/A.53 Researchers and clinicians have observed smoothing of skin overlying injected areas, which persists beyond the immediate neuromodulating effects of the neurotoxin54 and may result from local relaxation of transverse muscle cells and tissue remodeling due to muscle inactivity. In support, a statistically significant reduction in skin roughness was noted after BoNT/A treatment via quantitation of silicon replica scanning electron microscope images.54-56 It has been speculated that intradermal injection of BoNT/A preparations may modulate sebocyte lipogenesis (reducing sebum in sebocytes) and thereby minimize skin oiliness and pore size; the observed clinical effects on sebum could be due to a fibroblast growth factor receptor–binding mechanism of action, with a direct impact on sebocytes.57
Table 1. Approved US and European BoNT/A Neuromodulators With Facial Aesthetic Indications4,5,21,38,58-72
| Toxin Name (Non-proprietary) | First Approval Year (Country) | Manufacturer | Indications |
| OnabotulinumtoxinA | 1989 (US) | Allergan Aesthetics, an AbbVie Company | Facial aesthetic: Forehead lines (US, Europe) Glabellar lines (US, Europe) Lateral canthal lines (US, Europe) Therapeutic: Blepharospasm (US, Europe) Strabismus (US) Cervical dystonia (US, Europe) Axillary hyperhidrosis (US, Europe) Adult upper limb spasticity (US, Europe) Adult lower limb spasticity (US, Europe) Chronic migraine (US, Europe) Neurogenic detrusor Overactivity (US, Europe) Overactive bladder (US, Europe) Pediatric upper limb spasticity (US) Pediatric lower limb spasticity (US) Pediatric dynamic equinus foot deformity (Europe) Hemifacial spasm (Europe) |
| AbobotulinumtoxinA | 1991 (Europe) | Ipsen Ltd. | Facial aesthetic: Glabellar lines (US, Europe) Lateral canthal lines (Europe) Therapeutic: Adult upper limb spasticity (US, Europe) Adult lower limb spasticity (US, Europe) Blepharospasm (Europe) Cervical dystonia (US, Europe) Pediatric upper limb spasticity (US, Europe) Pediatric lower limb spasticity (US) Pediatric dynamic equinus foot deformity(Europe) Hemifacial spasm (Europe) Axillary hyperhidrosis (Europea) |
| IncobotulinumtoxinA | 2006 (Europe) | Merz Pharmaceuticals, LLC | Facial aesthetic: Glabellar lines (US, Europe) Lateral canthal lines (Europe) Forehead lines (Europe) Therapeutic: Adult upper limb spasticity (US, Europe) Blepharospasm (US, Europe) Cervical dystonia (US, Europe) Hemifacial spasm (Europe) Chronic sialorrhea (US, Europe) Pediatric upper limb spasticity, excluding spasticity caused by cerebral palsy (US) |
| PrabotulinumtoxinA | 2019 (US, Europe)b | Daewoong Pharmaceutical/Evolus, Inc. | Facial aesthetic: Glabellar lines (US, Europe) Therapeutic: None |
b2013 in Korea.
Summary
BoNT/A inhibits the release of the neurotransmitter acetylcholine at neuromuscular junctions by cleaving the SNAP-25 protein essential to its vesicular release, causing temporary, localized chemical denervation and muscle paralysis. The medical community serendipitously discovered the aesthetic benefits of injecting botulinum toxin into facial muscles, modulating the activity of muscles involved in wrinkle development and smoothing of the skin. Today, there are multiple formulations of botulinum toxin with different cosmetic and medical indications, providing facial aesthetic applications and benefits.
Acknowledgments
Writing and editorial assistance was provided to the authors by Regina Kelly, MA, of Peloton Advantage, LLC, an OPEN Health company, and funded by AbbVie, North Chicago, IL.
References
- Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev2000;80(2):717-766.
- Pirazzini M, Rossetto O, Eleopra R, et al. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev2017;69(2):200-235.
- Bak DH, Choi MJ, Lee E, et al. A comparison study of prabotulinumtoxinA vs onabotulinumtoxinA in myostatin-deficient mice with muscle hypertrophy. Basic Clin Pharmacol Toxicol2019;124(4):491-499.
- von Berg L, Stern D, Pauly D, et al. Functional detection of botulinum neurotoxin serotypes A to F by monoclonal neoepitope-specific antibodies and suspension array technology. Sci Rep2019;9(1):5531.
- Steward L, Brin MF, Brideau-Andersen A. Novel native and engineered botulinum neurotoxins. Handb Exp Pharmacol2020.
- Winter L, Spiegel J. Botulinum toxin type-A in the treatment of glabellar lines. Clin Cosmet Investig Dermatol2009;3:1-4.
- Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology2015;95(1-2):65-69.
- Jaspers GW, Pijpe J, Jansma J. The use of botulinum toxin type A in cosmetic facial procedures. Int J Oral Maxillofac Surg2011;40(2):127-133.
- Burke GS. The occurrence of Bacillus botulinus in nature. J Bacteriol1919;4(5):541-553.
- Jabbari B. History of botulinum toxin treatment in movement disorders. Tremor Other Hyperkinet Mov (N Y)2016;6:394.
- Clark RP, Berris CE. Botulinum toxin: a treatment for facial asymmetry caused by facial nerve paralysis. Plast Reconstr Surg1989;84(2):353-355.
- Monheit GD, Pickett A. AbobotulinumtoxinA: a 25-year history. Aesthet Surg J2017;37(suppl 1):S4-s11.
- Scott AB, Kennedy RA, Stubbs HA. Botulinum A toxin injection as a treatment for blepharospasm. Arch Ophthalmol1985;103(3):347-350.
- Hauser RA. Botulinum toxin injections in plastic surgery. 2019. Available at: https://emedicine.medscape.com/article/1271380-overview. Accessed August 26, 2020.
- Kaltreider SA, Kennedy RH, Woog JJ, et al. Cosmetic oculofacial applications of botulinum toxin: a report by the American Academy of Ophthalmology. Ophthalmology2005;112(6):1159-1167.
- Carruthers A, Kane MA, Flynn TC, et al. The convergence of medicine and neurotoxins: a focus on botulinum toxin type A and its application in aesthetic medicine–a global, evidence-based botulinum toxin consensus education initiative: part I: botulinum toxin in clinical and cosmetic practice. Dermatol Surg2013;39(3 Pt 2):493-509.
- Carruthers A, Carruthers J. Botulinum Toxin: Procedures in Cosmetic Dermatology Series. 3rd ed. New York, NY: Saunders; 2013.
- Ashenberg K. The real history behind the birth of botox. 2017. Available at: https://www.readersdigest.ca/health/beauty/birth-botox/. Accessed February 2, 2019.
- Blitzer A, Brin MF, Keen MS, et al. Botulinum toxin for the treatment of hyperfunctional lines of the face. Arch Otolaryngol Head Neck Surg1993;119(9):1018-1022.
- Gart MS, Gutowski KA. Overview of botulinum toxins for aesthetic uses. Clin Plast Surg2016;43(3):459-471.
- Botox [package insert]. Madison, NJ: Allergan USA; 2019.
- Coleman K. Botulinum toxin: mode of action and serotypes. Botulinum Toxin in Facial Rejuvenation. 2nd ed. Philadelphia, PA: Elsevier, Inc.; 2019:2-7.
- Rossetto O, Seveso M, Caccin P, et al. Botulinum neurotoxins are metalloproteases specific for SNARE proteins involved in neuroexocytosis. Curr Probl Dermatol2002;30:117-125.
- Brin MF. Botulinum toxins: pharmacology, immunology, and current developments. In: Benedetto AV, ed. Botulinum Toxins in Clinical Aesthetic Practice. Vol 1. 3rd ed. Boca Raton, FL: CRC Press; 2017:6-20.
- Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics2014;8:227-241.
- Inoue K, Fujinaga Y, Watanabe T, et al. Molecular composition of Clostridium botulinum type A progenitor toxins. Infect Immun1996;64(5):1589-1594.
- Sharma SK, Singh BR. Hemagglutinin binding mediated protection of botulinum neurotoxin from proteolysis. J Nat Toxins1998;7(3):239-253.
- Sharma SK, Singh BR. Enhancement of the endopeptidase activity of purified botulinum neurotoxins A and E by an isolated component of the native neurotoxin associated proteins. Biochemistry2004;43(16):4791-4798.
- Simpson LL. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol2004;44:167-193.
- Vaidyanathan VV, Yoshino K, Jahnz M, et al. Proteolysis of SNAP-25 isoforms by botulinum neurotoxin types A, C, and E: domains and amino acid residues controlling the formation of enzyme-substrate complexes and cleavage. J Neurochem1999;72(1):327-337.
- Myobloc [package insert]. Louisville, KY: Solstice Neurosciences; 2019.
- Fernandez-Salas E, Wang J, Molina Y, et al. Botulinum neurotoxin serotype A specific cell-based potency assay to replace the mouse bioassay. PLoS One2012;7(11):e49516.
- Hunt T, Rupp D, Shimizu G, et al. Characterization of SNARE cleavage products generated by formulated botulinum neurotoxin type-a drug products. Toxins (Basel)2010;2(8):2198-2212.
- Burstein R, Blumenfeld AM, Silberstein SD, et al. Mechanism of action of onabotulinumtoxinA in chronic migraine: a narrative review. Headache2020;60(7):1259-1272.
- Burstein R, Zhang X, Levy D, et al. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia2014;34(11):853-869.
- Flynn TC. Botulinum toxin: examining duration of effect in facial aesthetic applications. Am J Clin Dermatol2010;11(3):183-199.
- Rupp D, Nicholson G, Canty D, et al. OnabotulinumtoxinA displays greater biological activity compared to incobotulinumtoxinA, demonstrating non-interchangeability in both in vitro and in vivo assays. Toxins (Basel)2020;12(6):393.
- Botox [summary of product characteristics]. Bucks, UK: Allergan; 2020.
- Hunt T, Clarke K. Potency evaluation of a formulated drug product containing 150-kd botulinum neurotoxin type A. Clin Neuropharmacol2009;32(1):28-31.
- Brown M, Nicholson G, Ardila MC, et al. Comparative evaluation of the potency and antigenicity of two distinct BoNT/A-derived formulations. J Neural Transm (Vienna)2013;120(2):291-298.
- Kutschenko A, Manig A, Reinert MC, et al. In-vivo comparison of the neurotoxic potencies of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA. Neurosci Lett2016;627:216-221.
- Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon2020;175:64-68.
- Small R. Botulinum toxin injection for facial wrinkles. Am Fam Physician2014;90(3):168-175.
- Qaqish C. Botulinum toxin use in the upper face. Atlas Oral Maxillofac Surg Clin North Am2016;24(2):95-103.
- Warren H, Welch K, Coquis-Knezek S. AbobotulinumtoxinA for facial rejuvenation: what affects the duration of efficacy? Plast Surg Nurs2020;40(1):37-44.
- de Sanctis Pecora C. One21: A novel, customizable injection protocol for treatment of the forehead with incobotulinumtoxinA. Clin Cosmet Investig Dermatol2020;13:127-136.
- Rzany BJ, Ascher B, Avelar RL, et al. A multicenter, randomized, double-blind, placebo-controlled, single-dose, phase III, non-inferiority study comparing prabotulinumtoxinA and onabotulinumtoxinA for the treatment of moderate to severe glabellar lines in adult patients. Aesthet Surg J2020;40(4):413-429.
- de Maio M, Swift A, Signorini M, et al. Facial assessment and injection guide for botulinum toxin and injectable hyaluronic acid fillers: focus on the upper face. Plast Reconstr Surg2017;140(2):265e-276e.
- Sapra P, Demay S, Sapra S, et al. A single-blind, split-face, randomized, pilot study comparing the effects of intradermal and intramuscular injection of two commercially available botulinum toxin A formulas to reduce signs of facial aging. J Clin Aesthet Dermatol2017;10(2):34-44.
- Lee DH, Jin SP, Cho S, et al. RimabotulinumtoxinB versus onabotulinumtoxinA in the treatment of masseter hypertrophy: a 24-week double-blind randomized split-face study. Dermatology2013;226(3):227-232.
- Kapoor KM, Chatrath V, Anand C, et al. Consensus recommendations for treatment strategies in Indians using botulinum toxin and hyaluronic acid fillers. Plast Reconstr Surg Glob Open2017;5(12):e1574.
- Hoerter JE, Patel BC. Anatomy, head and neck, platysma. StatPearls. Treasure Island, FL: StatPearls Publishing; 2019.
- Humphrey S, Jacky B, Gallagher C. Preventive, cumulative effects of botulinum toxin type A in facial aesthetics. Dermatolog Surg2017;43(suppl 3):S244-S251.
- Carruthers A, Carruthers J, Lei X, et al. OnabotulinumtoxinA treatment of mild glabellar lines in repose. Dermatol Surg2010;36(suppl 4):2168-2171.
- Carruthers A, Carruthers J, Fagien S, et al. Repeated onabotulinumtoxinA treatment of glabellar lines at rest over three treatment cycles. Dermatol Surg2016;42(9):1094-1101.
- Dessy LA, Mazzocchi M, Rubino C, et al. An objective assessment of botulinum toxin A effect on superficial skin texture. Ann Plast Surg2007;58(5):469-473.
- You H, Chernavsky A, Grando S, et al. Botulinum neurotoxin type A (BoNT/A) modulates sebocyte lipogenesis [poster]. Presented at: Biennial TOXINS; January 16-19, 2019; Copenhagen, Denmark.
- Frenkl TL, Rackley RR. Injectable neuromodulatory agents: botulinum toxin therapy. Urol Clin North Am2005;32(1):89-99.
- Rasetti-Escargueil C, Lemichez E, Popoff MR. Variability of botulinum toxins: challenges and opportunities for the future. Toxins (Basel)2018;10(9):374.
- Park JY, Sunga O, Wanitphakdeedecha R, et al. Neurotoxin impurities: a review of threats to efficacy. Plast Reconstr Surg Glob Open2020;8(1):e2627.
- Cheon HI, Jung N, Won CH, et al. Efficacy and safety of prabotulinumtoxin A and onabotulinumtoxin A for crow’s feet: a phase 3, multicenter, randomized, double-blind, split-face study. Dermatol Surg2019;45(12):1610-1619.
- Frevert J, Ahn KY, Park MY, et al. Comparison of botulinum neurotoxin type A formulations in Asia. Clin Cosmet Investig Dermatol2018;11:327-331.
- Camargo CHF, Teive HAG. Use of botulinum toxin for movement disorders. Drugs Context2019;8:212586.
- Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol2014;7(2):31-39.
- Ferrari A, Manca M, Tugnoli V, et al. Pharmacological differences and clinical implications of various botulinum toxin preparations: a critical appraisal. Funct Neurol2018;33(1):7-18.
- Dysport for Injection [package insert]. Wrexham, UK: Ipsen Biopharm Ltd.; 2020.
- Xeomin [package insert]. Raleigh, NC: Merz Pharmaceuticals; 2020.
- Bocouture [summary of product characteristics]. Herts, UK: Merz Pharma UK; 2018.
- Vistabel Package Leaflet: Information for the User. 2020. Available at: https://www.medicines.ie/medicines/vistabel-34207/patient-info. Accessed October 2, 2020.
- Nuceiva [summary of product characteristics]. Dublin, Ireland: Evolus Pharma; 2020.
- Dysport [summary of product characteristics]. Berkshire, UK: Ipsen; 2020.
- Xeomin [summary of product characteristics]. Herts, UK: Merz Pharma; 2020.
